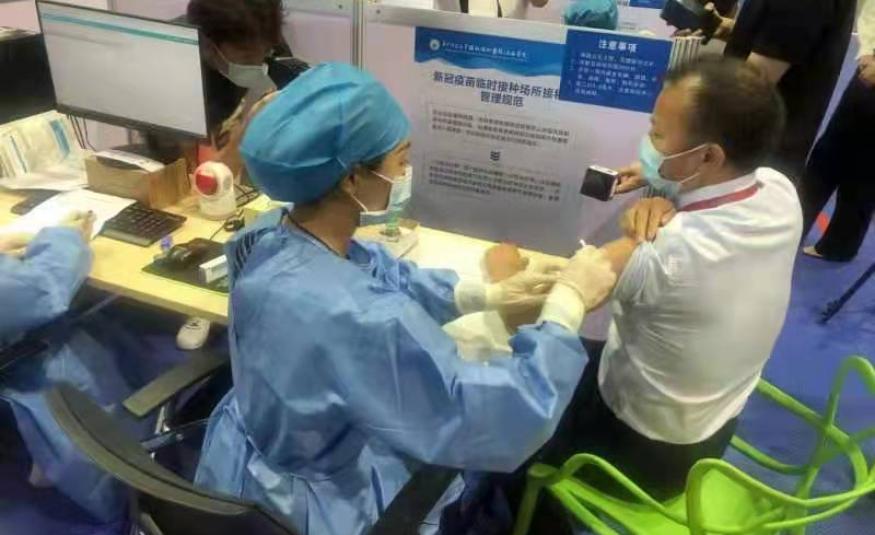
KUALA LUMPUR 4 June – YTB Healthcare Sdn Bhd (YTBH), a subsidiary of Yong Tai Bhd, is set to roll out the Phase III clinical trial in Malaysia after the National Pharmaceutical Regulatory Agency (NPRA) granted its approval for the Clinical Trial Import License (CTIL). This follows the earlier approval received from the Medical Review Ethics Committee (MREC).
The rollout makes Yong Tai, the first private company to conduct Phase III clinical trial for Covid-19 vaccine in Malaysia, and the successful completion of the trial would offer great confidence to the Malaysian population to be vaccinated.
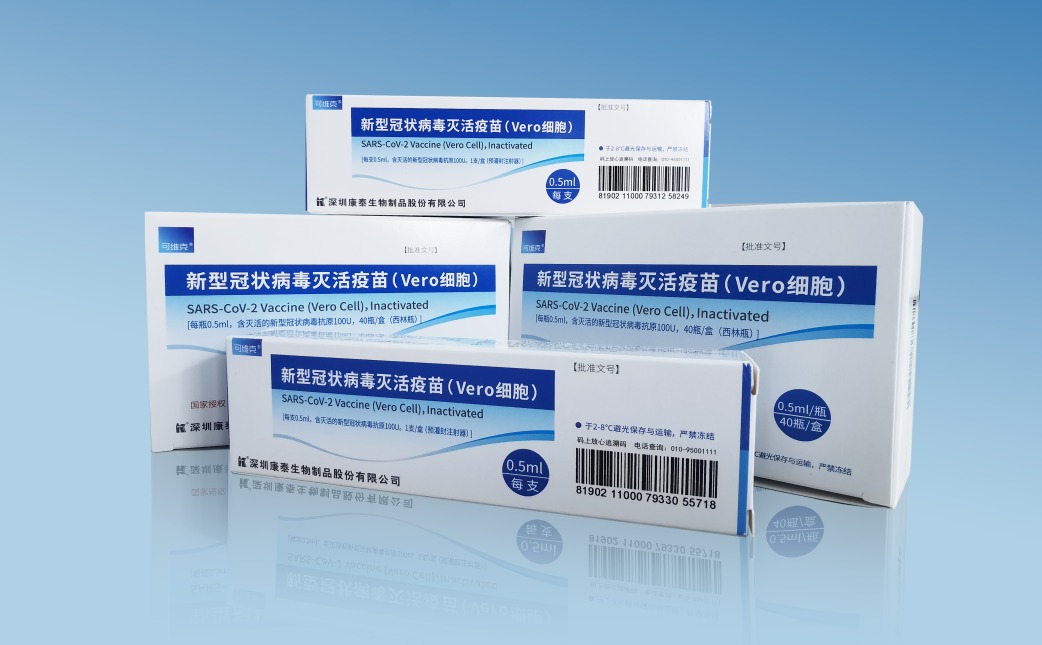
It also coincides with the Emergency Use Authorisation granted by the Government of China recently for the inactivated COVID-19 vaccine (KCONVAC) developed by the Group’s strategic partner, Shenzhen Kangtai Biological Products Co., Ltd. (SZKT).
SZKT has also commenced its KCONVAC vaccination programme in China, with its first batch of vaccination programme of more than 500,000 doses, on 1 June 2021.
Yong Tai’s Chief Executive Officer and Executive Director Datuk Wira Boo Kuang Loon said, Yong Tai are proud to be the first private company to conduct the Phase III clinical trial for COVID-19 vaccine in Malaysia.
He said, SZKT has already undergone the Phase I and II clinical trials in China since last year with encouraging results, and with the approval now granted for emergency use in China, Yong Tai are cautiously optimistic that the results of our Phase III clinical trial in Malaysia will show promising results. All Malaysians are encouraged to participate in this clinical trial.
The rollout of the Phase III clinical trial comes as Malaysia is facing a huge battle against the COVID-19 pandemic.
The government has warned of dire consequences if the nation’s healthcare system collapses under the strain of increasing COVID-19 cases, while the country will start a 2-week national full lockdown from June 1 to 14 following the surge in COVID-19 cases.
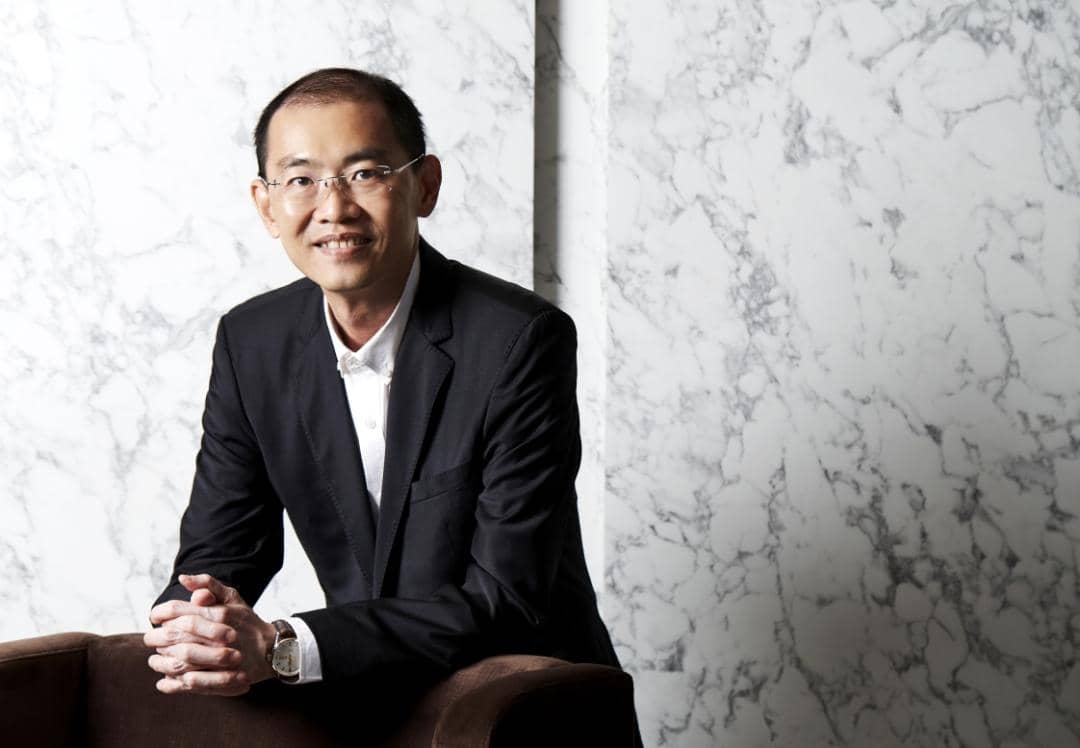
Given the sharp increase in COVID-19 cases that have put the country’s healthcare system at breaking point, Kuang Loon said that the Group would proceed with the application for the EUA in Malaysia, following the rollout of Phase III clinical trial.
“There is an urgent need to accelerate our vaccination programme, but the supply of vaccine in Malaysia is a key challenge. Following the rollout of Phase III clinical trial, we will proceed to apply for the EUA in Malaysia.
"As our strategic partner has already managed to obtain approval for emergency use in China, we think this will pave the way for us to obtain similar approval in Malaysia.
"We hope that the faster approval for emergency use in Malaysia will help to bridge the supply gap of the vaccine in the country as we are committed to provide 10 million doses plus another option of 10 million doses yearly by our partner,” said Kuang Loon, in a statement.
He added that the approval would also add another vaccine supply for the country, on top of the currently available vaccines – Pfizer, Astra Zeneca and Sinovac.
The resurgence of COVID-19 cases has been a concern in the region, as seen in other countries, including Thailand, Vietnam, Japan, India and others.
As these countries rushed to increase the procurement of vaccines, this could potentially affect the supply of vaccines, causing further delays in the rollout of the vaccination programme in the region, including Malaysia.
"Yong Tai hopes to contribute to ensure Malaysia can procure sufficient vaccines timely, in order to achieve herd immunity soonest and help Malaysians’ return to their normal life," said the statement. - DagangNews.com