KUALA LUMPUR 9 Nov – Solution Group Berhad, wholly owned subsidiary, Solution Biologics Sdn Bhd (SOLBIO), the ASEAN manufacturing partner of CanSino Biologics Inc’s (CanSino) single-dose Convidecia vaccine for COVID-19, is pleased to announce that they are expecting approval to sell Convidecia vaccine on the private market and administer as a booster shot soon.
SOLBIO submitted data showing the efficacy of Convidecia vaccine as a booster shot to the National Pharmaceutical Regulatory Agency (NPRA) demonstrating that taking a Convidecia booster shot six months after the first dose showed an increase of at least 8 times in antibody levels.
The submission of the data to the NPRA is in preparation for Convidecia vaccine to be authorised as a booster shot.
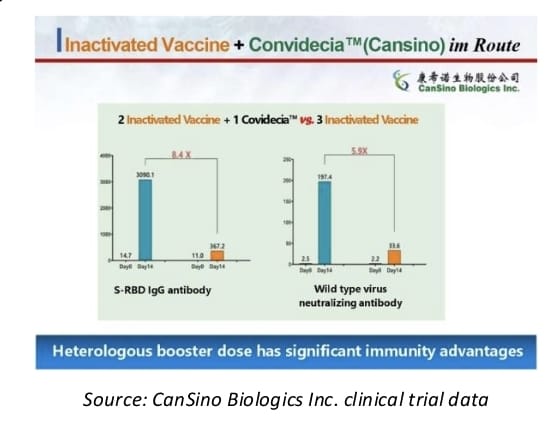
In a separate study by Jiangsu Province Centre of Disease Control and Prevention reported earlier, the data also showed that mixing of vaccine with Convidecia as a booster shot after taking a different two-dose inactivated vaccine has significant safety and efficacy advantage.
The results demonstrated that antibody levels from a Convidecia booster shot is 8.4 times higher in IgG antibody and 5.9 times higher in neutralizing antibody in comparison with a booster shot from an inactivated vaccine (As shown in picture below).
Compared to three-dose of inactivated vaccines, the two-dose inactivated vaccines plus one dose of Convidecia vaccine reduced the adverse event significantly. In addition, the data also revealed that Convidecia is effective against the highly infectious Delta variant of COVID-19.
Deputy Group Managing Director of SGB, Dato’ Dr. Mohd Nazlee Kamal said, “As soon as we get approval from the Ministry of Health (MOH), we will be expanding our vaccine distribution to the private market, such as hospitals, clinics, the corporate and manufacturing sectors in Malaysia.
‘’We also hope to get the NPRA approval for Convidecia to be used as a booster shot in November. Once it is approved, it will be an additional option for those who originally received a two-dose inactivated vaccine as the Jiangsu study shows that Convidecia is safe and highly efficacious as a booster”.
“The technical working group from the Special Committee for Ensuring Access to COVID-19 Vaccine Supply (JKJAV) recommends the use of different vaccines to boost vaccine efficacy as practiced by other countries.
‘’We have more than enough capacity to supply both primary and booster doses to fulfill the market demand not just for Malaysia but also the ASEAN region”.
SOLBIO, under an agreement with MOH, is supplying 3.5 million doses of Convidecia. To date, SOLBIO has completed the delivery of 2.6 million doses of the COVID-19 vaccine.
Another 300,000 doses are arriving on the 12th of November and the balance of 600,000 doses to be delivered by December.
With the completion of the MOH contract, the sales from the private sector, and the contribution from other business segments, Solution is expecting a turnover of approximately RM300 million with a profit margin of 10 to 15 % for the financial year ending 2021. – DagangNews.com










